|
Health Care Enforcement Defense Practice | Health Law & Policy Matters blog
February 2014
Mintz Levin Health Care Qui Tam Update
Recent Developments & Unsealed False Claims Act (FCA) Cases
By Kevin McGinty, Stefanie Abhar, Sean Grammel, and Stephanie Willis
- We have identified 23 health care–related qui tam cases unsealed since last month’s Qui Tam Update. Only six of those cases were filed in 2013. The majority (13 cases) were filed in 2011 or 2012, with the remainder dating back as far as February 2007.
- These cases were filed in 12 states. A number of cases were filed in historically active jurisdictions for FCA cases, including the Eastern District of Pennsylvania and the District of Massachusetts.
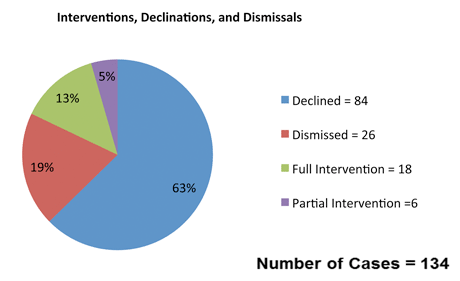
- The government declined to intervene in most of the cases that we reviewed. Among the unsealed cases where the publicly available filings included the government’s decision on intervention, the government intervened, or intervened in part, in only five cases.
- Subject matter of claims:
- 10 of the 23 recently unsealed cases involved both state and federal claims.
- 4 of the 23 reviewed cases involved alleged violations of the Anti-Kickback Statute (AKS).
- 5 of the 23 reviewed cases (approximately 22%) were filed against pharmaceutical manufacturers or medical device manufacturers.
- 8 of the 23 reviewed cases (approximately 35%) asserted claims against hospitals, hospital management companies, and community health centers.
- Identity of relators:
- More than 60% of the relators in these 23 cases were employees or former employees of the defendants.
- One case was filed by a relator who was a plaintiff in a personal injury action against the defendant.
United States ex rel. Oughatiyan v. IPC The Hospitalist Company Inc., Civ. No. 09-C-5418 (N.D. Ill.).
Complaint Filed: September 1, 2009
Complaint Unsealed: December 5, 2013
Intervention Status: The United States intervened, but Illinois and the other 12 plaintiff states declined to intervene.
Claims: Defendants allegedly encouraged the filing of upcoded claims for services in inpatient and long-term care facilities to federal health care programs.
Name of Relator: Dr. Bijan Oughatiyan
Defendants’ Business: National hospitalist independent contractor company and its local subsidiaries “employing physicians and other health care providers who work in more than 1,300 facilities in 28 states.”1 Hospitalists are physicians who assist in directing and coordinating inpatient care from admission to discharge, and only work in hospitals or long-term care facilities.
Relator’s Relationship to Defendants: Relator is a former employee/independent contractor of defendant.
Relator’s Counsel: Goldberg Kohn Ltd. (Chicago, IL)
Summary of Case: Relator alleges that IPC The Hospitalist Company (IPC) engaged in the following schemes to cause its employed hospitalists to bill for the services they rendered at the highest reimbursement levels even though such codes were inappropriate, a practice called “upcoding.” The lawsuit contends that IPC trained its physicians to bill at the highest levels without regard to the actual complexity of the services provided. Additionally, IPC allegedly tracked the coding statistics of its hospitalists and used the results to pressure hospitalists to upcode their services to achieve productivity and profit goals. As a result of these practices, according to the relator, the medical documentation of the actual work done did not support the billing records submitted by the hospitalists.
Current Status: Ongoing
Reasons to Watch: This case involves a defendant named in a qui tam case that was unsealed in 2013 (United States ex rel. Ziaei v. IPC The Hospitalist Company Inc., et al., Civ. No. 2:12-cv-01918 (D. Nev.)). Although it was filed later than Oughatiyan, the Ziaei complaint included very similar allegations. The facts alleged in the Ziaei complaint were not as well developed as those asserted in the Oughatiyan complaint, and consequently the Ziaei complaint was voluntarily dismissed. Companies that operate nationally should be aware that their legal exposure is national in scope as well. Thus, counsel concerned about certain practices may wish to conduct internal investigations, consider remedial strategies, and seek to reach global settlements to resolve cases efficiently. Significantly, the case was investigated by the interagency Health Care Fraud Prevention and Enforcement Action Team (HEAT).
United States ex rel. Nevyas v. Allergan, Inc., No. 2:09cv432 (E.D. Pa).
Complaint Filed: January 30, 2009 (Second Amendment Complaint Filed September 27, 2010)
Complaint Unsealed: December 16, 2013
Intervention Status: Unclear from docket
Claims: The relators assert that the defendant caused the submission of claims for payment for prescription drugs induced by illegal kickbacks in violation of the FCA, as well as analogous false claims statutes of 19 states (California, Delaware, Florida, Illinois, Indiana, Louisiana, Massachusetts, Michigan, Montana, Nevada, New Hampshire, New Jersey, New Mexico, New York, Oklahoma, Rhode Island, Texas, Virginia, and Wisconsin) and the District of Columbia.
Relators’ Names: Herbert J. Nevyas, M.D.; Anita Nevyas-Wallace, M.D.
Defendant’s Business: The defendant is an international biopharmaceutical company.
Relators’ Counsel: Pietragallo, Gordon, Alfano, Bosick & Raspanti LLP (Philadelphia, PA)
Relators’ Relationship to Defendant: The relators are third-party physicians who claim they were offered the alleged inducements by the defendant.
Current Status: Ongoing
Summary of Case: The relators allege that the defendant violated the AKS by offering inducements to ophthalmologists and optometrists to prescribe the defendant’s exclusive chronic dry-eye prescription drug, Restasis®. The alleged inducements were in the form of (i) free, on-demand business advisory and consulting services; (ii) free memberships to the company’s restricted access website; (iii) invitations to and payment of expenses related to advisory board meetings; and (iv) offers to fund independent research. The relators also allege that the defendant’s conduct resulted in the submission of false claims to federal and state health care programs.
Reasons to Watch: This case is noteworthy for at least two reasons. First, shortly before the relators filed their Second Amended Complaint, Allergan entered into a five-year Corporate Integrity Agreement (CIA) with the Department of Health and Human Services, Office of Inspector General (HHS-OIG) in connection with the settlement of an unrelated criminal investigation and qui tam action.2 The relators’ complaint suggests that at least some of the challenged conduct may have occurred while the CIA was in place. Although it is unclear from the docket if the government will intervene in this case, the existence of the CIA may factor into that decision and could potentially raise additional issues for Allergan. Second, apart from the potential implications for Allergan, this case brings to light important compliance issues for pharmaceutical companies seeking to expand their business through business relationships with physicians.
United States ex rel. Fife v. Lymphedema and Wound Institute, Inc., Civ. No. 04:11-CV-271 (S.D. Tex.).
Complaint Filed: September 22, 2011
Complaint Unsealed: November 25, 2013
Intervention Status: The United States intervened.
Claims: Defendants allegedly submitted false claims for treatment of lymphedema
Name of Relator: Dr. Caroline Fife
Defendants’ Businesses: The individual defendants are the executives and owners of the defendant company and its affiliates, whose employees provide physical therapy and treatment for lymphatic disease. The individual defendants also managed and operated a network of sleep-study clinics.
Relator’s Relationship to Defendants: Relator is a competing physician and professor at the University of Texas who often treated patients who had stopped receiving treatments from defendants’ facilities.
Relator’s Counsel: Ahmad, Zavitsanos, Anaipakos, Alavi & Mensing P.C. (Houston, TX)
Summary of Case: The relator alleged that the defendants improperly used unqualified massage therapists at its eight Houston-area locations to render physical therapy treatments to lymphedema patients. The complaint also contended that the defendants submitted false claims for lymphedema treatments and supplies that were never rendered to patients and that it billed for unnecessary services in excess of those permitted by Medicare billing policies. Lastly, the relator alleged that the individual defendants used a similar scheme to inflate the billings for services that were rendered at their sleep clinics.
Current Status: The parties settled the claims related to lymphedema treatments for $4.3 million. Additionally, the defendant company’s founder and CEO voluntarily submitted to a 10-year exclusion from federal health benefit programs and the defendant company entered into a five-year Corporate Integrity Agreement (CIA) as of June 25, 2013.
Reasons to Watch: Although the amount of the settlement — $4.3 million — is relatively modest when compared with the $165 million in fraudulent Medicare billings alleged in the complaint, the voluntary exclusion of the defendant company’s CEO from participation in federal health care programs is severe, as an excluded individual will likely find it difficult to continue working in the health care industry.
In addition to our regular review of recently unsealed qui tams, this edition of the Qui Tam Update also provides additional information about some of the cases that we reviewed during the past six months.
We are able to provide updates on three cases that were featured in our prior newsletters:
- The parties in two of the previously profiled cases appear to have settled out of court. In United States v. Amerigroup Corp., the court granted the relator’s motion to voluntarily dismiss the case on December 17, 2013.3 In Theis v. Northwestern University, a minute entry on the docket from December 4, 2013, indicates that counsel announced the settlement of the case.4
- In contrast, several pleadings have been filed in United States v. Diagnostic Physicians Group since we reviewed it in August 2013.5 Diagnostic Physicians Group involved false claims allegations, including some premised on false certification of compliance with the AKS and the Physician Self-Referral Law (Stark Law). In August, we noted the substantial dollar value of the claims allegedly submitted to and reimbursed by federal health care programs. Since the United States filed its complaint as intervenor on August 7, 2013, the following developments have occurred:
- all of the defendants moved to dismiss the United States’ claims on October 7, 2013;
- the United States amended its complaint on October 30, 2013;
- the United States filed its Opposition to the defendants’ motion to dismiss on November 15, 2013; and
- four of the five defendants also moved to dismiss the relator’s case on December 30, 2013.
In its complaint, the United States intervened in the relator’s allegations under the FCA for presenting false claims under 31 U.S.C. § 3729(a)(1) and (a)(1)(A), using false statements to get false claims paid under 31 U.S.C. § 3729(a)(1)(B), and making reverse false claims under 31 U.S.C. § 3729(a)(7) and (a)(1)(G). The United States did not intervene on the relator’s claims involving the AKS or conspiracy allegations, but added two additional common-law claims: payment by mistake and unjust enrichment. In moving to dismiss the United States’ FCA claims, the defendants argued that the United States failed to allege the dates or amounts of the defendants’ purportedly false claims with sufficient particularity as required by Rule 9(b) of the Federal Rules of Civil Procedure. Additionally, the defendants moved to dismiss the United States’ common-law claims because of the lack of a predicate FCA claim and because the United States failed to articulate whether the claims arose under federal or state common law. Although substantial briefing has occurred in this case, the court has yet to rule on defendants’ motions.
Of the cases that informed the past six months’ updates, 134 cases have sufficient pleading and docket information to allow statistical analysis of concerning parties, status, and disposition. 6 Based on our review of those 134 cases, we have calculated the following informative statistics about current qui tam cases:
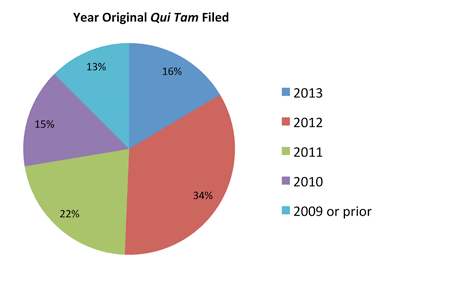
The following chart illustrates by percentage when reviewed cases were originally filed. As this chart shows, only 16% of the cases we reviewed were filed in 2013. Cases filed in 2012 make up the largest portion of the cases unsealed in the past six months, while fully 50% of cases that we reviewed date from 2011 and earlier.
The charts that follow show where our reviewed cases were filed, with New York being the busiest state and the Eastern District of Pennsylvania (Philadelphia), not surprisingly, the busiest district (relators and their lawyers have long believed that it is a favorable district in which to bring such suits).
| |
Top States
State(s) |
Number |
New York |
16 |
Florida, Texas &
California |
13 |
Pennsylvania |
11 |
Massachusetts |
9 |
Illinois |
8 |
|
|
Top Districts
State(s) |
Number |
E.D. Pennsylvania |
10 |
M.D. Florida |
9 |
C.D. California,
N.D. Illinois & S.D.
New York |
7 |
E.D. New York & S.D.
Texas |
5 |
E.D. California,
S.D. Florida & W.D.
Texas |
7 |
|
|
Attorneys from Mintz Levin’s Health Care Enforcement Defense Practice have done an in-depth review of the overarching trends to watch from 2013 and our predictions of what is to come is in Health Care Enforcement in 2013: A Year in Review. But, in the context of our review of the past six months’ of qui tam actions, we continue to see unsealed cases that focus on skilled nursing facilities, durable medical equipment suppliers, and pharmaceutical companies. Also, independent contracting entities, such as hospitalist and anesthesiologist corporations, are increasingly being sued by qui tam relators. Finally, the number of auditors, accountants, and high-ranking corporate executives who are becoming qui tam relators seems to be growing, and they are alleging evermore complex and sophisticated schemes of fraud that reflect their familiarity with and understanding of the health care enforcement laws.
Mintz Levin’s Health Care Enforcement Defense Practice is comprised of health law, employment, and white collar defense attorneys with experience in government investigations and health care regulatory compliance matters. We regularly help clients conduct internal investigations designed to detect and correct problems before the government becomes involved. We have represented clients in federal and state government investigations and litigation across the country in matters initiated by the Criminal and Civil Divisions at the Department of Justice, United States Attorneys, the Office of Inspector General for the Department of Health and Human Services, the Drug Enforcement Administration, State Attorneys General, Medicare and Medicaid contractors, and the 50 Medicaid Fraud Control Units. We have helped clients avoid potentially ruinous civil fines, incarceration, other criminal and administrative penalties, and exclusion by combining our regulatory knowledge with our investigative, employment-related and litigation capabilities.
* * *
Endnotes
1 Press Release, U.S. Department of Justice, “Government Intervenes in False Claims Lawsuit Against Ipc the Hospitalist Co., Inc. Alleging Overbilling of Physician Services,” (Dec. 9, 2013).
2 Press Release, U.S. Department of Justice, “Allergan Agrees to Plead Guilty and Pay $600 Million to Resolve Allegations of Off-Label Promotion of Botox®,” (Sept. 1, 2010).
3 Kevin McGinty, Brian Dunphy, and Samantha Kingsbury, Mintz Levin Health Care Qui Tam Update, (October 2013), http://www.mintz.com/newsletter/2013/Newsletters/3502-1013-NAT-LIT/index.html.
4 Kevin McGinty, Matthew Levitt, and Aaron Tidman, Mintz Levin Health Care Qui Tam Update, (September 2013), http://www.mintz.com/newsletter/2013/Newsletters/3406-0913-NAT-LIT/index.html.
5 Kevin McGinty, Samantha Kingsbury, and Stephanie Willis, Mintz Levin Health Care Qui Tam Update, (August 2013), http://www.mintz.com/newsletter/2013/Newsletters/3157-0613-NAT-LIT/index.html.
6 Certain cases were only partially unsealed, thus resulting in limitations on the information available for analysis.
|
|


