FDA Continues Push to Improve Food Labeling Practices in the United States
In September 2022, former President Biden convened the White House Conference on Hunger, Nutrition, and Health, during which the White House introduced its National Strategy on Nutrition and Health (National Strategy). The National Strategy called for creating more accessible food labeling practices to empower consumers to make healthier choices, among other laudable public health-focused goals. Prior to the January 2025 transition from the Biden to the Trump administration, the Food and Drug Administration (FDA) took concrete steps to address this particular National Strategy priority through both formal rulemaking and informal guidance. This blog post summarizes FDA's actions at the end of the Biden administration intended to modernize food labeling practices and move them forward in today’s more consumer-focused marketplace.
Proposed Rule for Front-of-Package Nutrition Labeling
In the National Strategy, the development of front-of-package (FOP) labeling schemes was discussed as one way to promote equitable access to nutrition information and healthier choices. On January 16, 2025, FDA published in the Federal Register a proposed rule that would require a front-of-package nutrition label on packaged foods (Proposed Rule). The Proposed Rule would require manufacturers to add a "Nutrition Info" box on the principal display panel of each packaged food product, which would list the Daily Value (DV) percentage of saturated fat, sodium, and added sugars in a serving of that food. The DV percentage would list how much of the nutrient in a serving contributes to a person's total daily diet. In addition, each of those nutrients would include corresponding “interpretative information” that would signal to consumers whether the food product contains a low, medium, or high amount of those nutrients. An example of the proposed FOP nutrition information graphic is below. And although the Proposed Rule would not require it, manufacturers could voluntarily include a calorie count on the front of the food package, per existing FDA regulations.
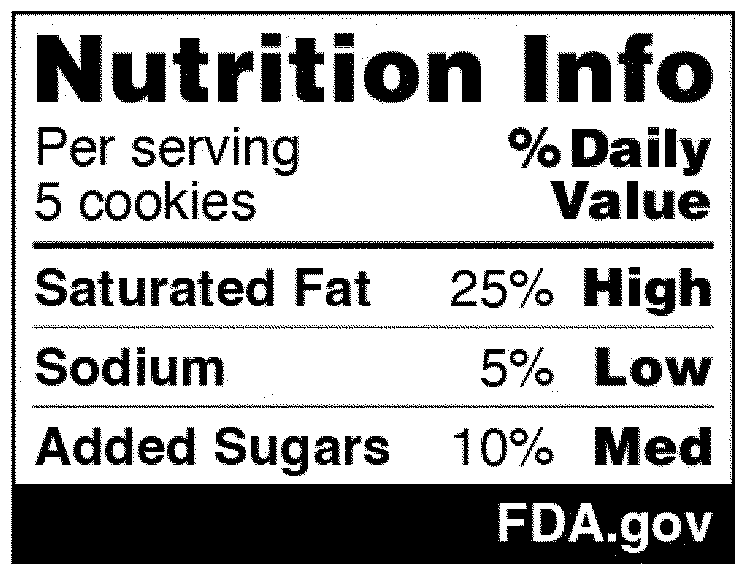 |
The Proposed Rule does deviate from certain suggestions made in the National Strategy, which advocated for FOP "star ratings" and "traffic light schemes" to promote equitable access to nutrition information. Specifically, the National Strategy considered how to best help consumers with lower nutrition literacy more readily identify foods that comprise a healthy diet. Instead of a front-of-packaging labeling system that would rely on imagery, however, FDA’s proposal opted for written information about the nutrients contained in the food. Both the preamble to the Proposed Rule and FDA’s press release announcing its publication explain that in focus groups conducted in 2022, participants reported confusion over the traffic light system in particular (e.g., when a food contained both nutrients that should be limited but also nutrients for which higher consumption is recommended) and that “the black and white Nutrition Info scheme with the percent [DV] performed best in helping consumers identify healthier food options.”
It will be interesting to see whether comments to the Proposed Rule will remark on FDA's choice of the written "Nutrition Info" box versus a FOP labeling system that would be more reliant on imagery. FDA is accepting comments on the Proposed Rule until May 16, 2025 (Docket FDA-2024-N-2910). As currently envisioned, if the proposal for FOP nutrition information is adopted, most food product manufacturers would have three years from the effective date to bring labels into compliance (smaller manufacturers would be given four years).
As a result of President Trump’s administrative freeze and new executive orders governing the work of regulatory agencies such as FDA, the fate of this Proposed Rule is currently uncertain. However, newly confirmed Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. has articulated that his agenda is to “make America healthy again” (MAHA) and the presidential MAHA Commission was recently established to begin informing the Administration’s work on Mr. Kennedy and President Trump’s priorities in this space. Although Mr. Kennedy did not address food labeling during his Senate confirmation hearings and the executive order creating the MAHA Commission does not speak directly to food labeling or nutrition information accessibility for consumers, interested stakeholders should monitor the upcoming work of the Commission – including whether any opportunities for public comments may be made available – as well as its future “Make Our Children Healthy Again Strategy” that is due in approximately six months. Further, under a deregulatory executive order signed on January 31, 2025, President Trump has directed agencies to eliminate 10 “regulations” for each new regulation to be promulgated, with the term “regulation” expansively defined to include memoranda, guidance documents, policy statements, and interagency agreements. This “one-in, 10-out” order may make the prospect of an FOP nutrition labeling final rule less likely, at least for the foreseeable future.
Final Rule for Use of The Term "Healthy" on Food Labeling
Another recent FDA action related to food labeling was the agency’s finalization of a proposed rule from 2022 that involved a lengthy public consultation and information collection process (see our prior coverage here). On December 27, 2024, FDA published in the Federal Register its Final Rule regarding the use of the term "healthy" in food labeling. The Final Rule updates the definition established 30 years ago for the nutrient content claim "healthy" to be used in food labeling. In President Biden’s National Strategy, one highlighted priority was ensuring that food packages bearing this claim align with current nutrition science and the Dietary Guidelines for Americans (Dietary Guidelines). To advance this goal, FDA was charged with updating the standards for when a company can use the "healthy" claim on its products (work on which was already ongoing at the agency), creating a symbol that can be used to reflect that the food is "healthy, and developing guidance on the use of Dietary Guideline statements on food labels.
The original regulatory definition of “healthy” (codified at 21 C.F.R. § 101.65(d)) sets limits on total fat, saturated fat, cholesterol, and sodium content should a food be labeled as healthy, and requires that the food contain at least 10% of the DV for vitamin A, vitamin C, calcium, iron, protein, and fiber. Under the Final Rule, total fat and dietary cholesterol are no longer factors to be considered when evaluating whether a food is eligible for this particular nutrient content claim. Instead, the agency has established limits on saturated fat, sodium, and added sugars in accordance with the Dietary Guidelines. Additionally, rather than focusing on vitamin A, vitamin C, calcium, iron, protein, and fiber, the Final Rule requires that the food product contain a certain amount of food from at least one of the food groups or subgroups recommended by the Dietary Guidelines, such as fruit, vegetables, grains, dairy, and proteins.
Perhaps most notably, the prior regulatory scheme allowed for foods that were high in added sugars, such as yogurts, breakfast cereals, and fruit snacks, to technically qualify as "healthy" despite not aligning with the definition of "nutrient-dense" foods from the Dietary Guidelines, which specifically applies to certain foods "when prepared with no or little added sugars, saturated fat, and sodium." Consistent with generally accepted nutritional best practices, the National Strategy also promoted lowering the sodium content in food and decreasing the consumption of added sugars –shared goals of new HHS Secretary Kennedy and the broader MAHA agenda.
The Final Rule does not establish a "healthy" symbol that can be used on food packaging, but FDA has indicated that this symbol may also be on the horizon. In its press release announcing the Final Rule, FDA noted that it is "continuing to develop" this symbol, adding that such a symbol would further FDA's goal of helping consumers more easily identify healthier food products.
The Final Rule’s effective date was changed by the Trump Administration from February 25, 2025 to April 28, 2025. The compliance date for manufacturers remains February 25, 2028.
Draft Guidance for Industry: Labeling of Plant-Based Alternatives to Animal-Derived Foods
Finally, while not specifically called out in the National Strategy, FDA has been working for several years to develop labeling recommendations for plant-based foods that are being developed and marketed as alternatives to conventional animal products. On January 7, 2025, FDA released the Draft Guidance for the Labeling of Plant-Based Alternatives to Animal Derived Foods (Draft Guidance), in response to the growing demand for plant-based food alternatives in the United States. According to the Plant-Based Food Association, 70% of Americans are consuming plant-based foods. The scope of the newly released guidance encompasses alternatives to poultry, meat, seafood, and dairy products that fall under FDA's jurisdiction. It expressly excludes plant-based milk alternatives, as separate guidance on that subject was released in February 2023.
The Draft Guidance notes that rather than simply identifying a product as a "plant-based" alternative food, the specific plant source should be disclosed on the food product’s label. This would enable consumers to make more informed choices about purchasing plant-based alternatives. For example, rather than labeling a plant-based cheese solely as such, the cheese's label should more clearly disclose "soy-based cheese" to reflect its primary ingredients. The Draft Guidance also recommends that if a plant-based alternative food is derived from several different plant sources, the primary plant sources should be identified in the food's name. The agency provides the examples of "Black Bean Mushroom Veggie Patties" and "Chia and Flax Seed Egg-less Scramble" to illustrate this concept. For labeling purposes, FDA also recommends companies avoid exclusively naming products with "vegan," "meat-free," or "animal-free."
Public comments on the Draft Guidance should be submitted by May 7, 2025 (Docket FDA-2022-D-1102).
Conclusion
One primary goal of the National Strategy was to empower Americans to make healthier, informed choices about their nutrition and food consumption. In the United States, diet-related diseases, such as hypertension, obesity, and diabetes, are on the rise. Under the Biden administration and the leadership of former Commissioner Dr. Robert Califf, FDA sought to fight these alarming trends and to improve public health by increasing access to nutritional information and promoting transparency in food labeling.
Further, while the Proposed Rule, Final Rule, and Draft Guidance all focus on labeling packaged food products that can be purchased in stores, it will be interesting to see how these initiatives influence FDA's recommendations for food labeling practices in online grocery shopping. On April 24, 2023, FDA published the notice Food Labeling in Online Grocery Shopping; Request for Information (Docket No. FDA-2023-N-0624-0002), which received 31 electronically submitted comments from various stakeholders, including grocer organizations, food scientists, and individual consumers. Indeed, the December 2024 press release for the Final Rule noted that FDA “has already entered into a partnership with Instacart to make it even easier for consumers to find products with the ‘healthy’ claim through online grocery shopping filters and a virtual storefront.” In the wake of the agency actions summarized in this post and the Instacart partnership, we wonder if FDA will move in the future to provide manufacturers and retailers with definitive guidance on online food labeling practices. We will be watching to see how FDA, as well as the work of the MAHA Commission and HHS Secretary Kennedy, may continue to improve food labeling practices in the future.

Regulatory Roundup: Important FDA Developments at the End of September 2022
October 10, 2022| Blog

FDA Faces Critical Deadlines in 2024, Even Without an Election Looming
March 6, 2024| Blog



