A Later-Filed, Later-Expiring Unrelated Patent is Not a Proper Reference Patent for an Obviousness-Type Double Patenting Rejection
The recent Patent Trial and Appeal Board (PTAB) decision Ex Parte Baurin[1] reversed an Examiner rejection for obviousness-type double patenting (ODP) in U.S. Application No. 17/135,529 over the later-filed, later-expiring unrelated U.S. Patent No. 10,882,922 (timeline below). The USPTO Examiners routinely reject patent applications for ODP over later-filed, later-expiring unrelated patents because Examiners use the pre-URAA[2] standard of issue date to determine the reference patent without consideration to whether the two cases have the same or different priority dates. This forces applicants to file unnecessary terminal disclaimers or abandon applications altogether. The reversal in Ex Parte Baurin is a welcome relief as the current application of ODP limits the claims an applicant can pursue in continuation applications. The USPTO should incorporate this decision into its ODP training material and update the standard used to determine the reference patent by focusing on filing date when the patents have different priority dates.
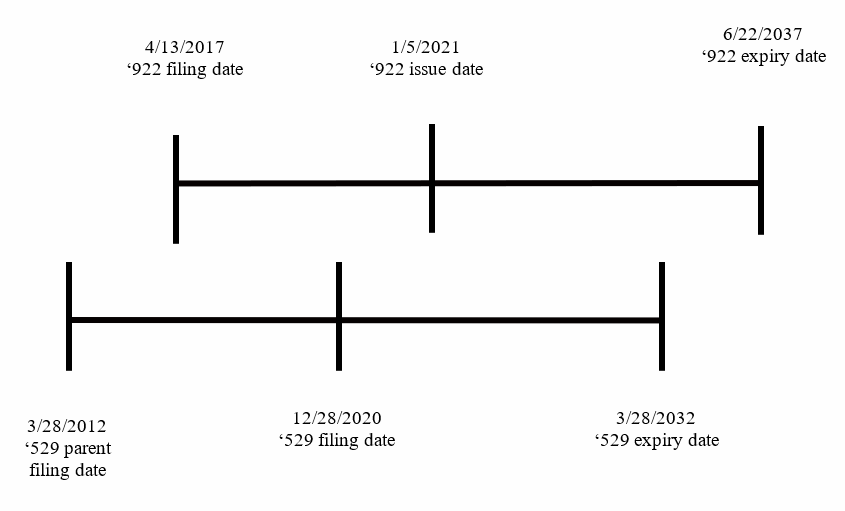
The claims at issue in Baurin recite an antibody-like binding protein comprising two polypeptide chains that form two antigen binding sites. The claims of the later-filed, later-expiring unrelated ’922 patent recite a trispecific construct that is alleged to contain the “instantly recited bispecific ‘cross-over’ constructs and linkers … except for the ‘swapping’ of the VL and VH domains.”[3] The Appellant argued the later-filed, later-expiring unrelated ’922 patent is not the proper reference patent because it has a later filing date, and expires later. In doing so, the Appellant pointed to the In re Cellect[4] decision which noted that “claims ‘are entitled to their full term, including … duly granted PTA, unless they are found to be later-filed obvious variations of earlier-filed … claims.”[5] The Appellant also argued that for patents with different priority dates, it is the expiration date that is the controlling factor, citing to Gilead Scis., Inc. v. Natco Pharma Ltd.[6] For post-URAA patents, expiration date is tolled by the first non-provisional filing date, so filing date is a stand-in for expiration date.
In finding that the later-filed, later-expiring unrelated ’922 patent cannot serve as the reference patent, the Board pointed to Allergan USA, Inc. v. MSN Labs. Private Ltd.,[7] noting that the “purpose of nonstatutory double patenting doctrine ‘is to prevent patentees from obtaining a second patent on a patentably indistinct invention to effectively extend the life of a first patent to that subject matter.’”[8] The Board also distinguished the facts in Gilead v. Natco and AbbVie Inc. v. Mathilda & Terence Kennedy Inst. of Rheumatology Trust [9], as the reference patent in both decisions was the earlier-filed, earlier-expiring patent rather than the later-filed, later-expiring unrelated patent in Baurin. In doing so, the Board found that because the later-filed, later-expiring unrelated ’922 patent was relied on for the reference patent, “the facts here compel a different conclusion.”[10]
Instead, the Board again pointed to the decision in Allergan, which noted that “[a]s the first-filed, first-issued patent in its family, it is the patent that sets the maximum period of exclusivity for the claimed subject matter and any patentably indistinct variants.”[11] The Board acknowledged Allergan involved patents with a shared priority date in contrast to the Baurin facts involving different priority dates. Still, the Board found the Allergan “reasoning compelling”, concluding that the earlier-filed, earlier-expiring ’529 application is the “first patent” and “not a second, later expiring patent for the same invention.”[12] This is consistent with the new rubric for ODP set forth in our recent article The New Rubric for Obviousness-Type Double Patenting, finding that the reference patent is the earlier-filed patent when the patents have different priority dates, and the reference patent is the earlier-issued patent when the patents have the same priority date.
The Examiner’s rejection in the ’529 application shows the pre-URAA standard of looking to the issue date to determine the proper reference patent continues to be applied, irrespective of whether the two patents have the same or different priority dates. As the Gilead decision shows, gamesmanship can result when issue date is relied on to determine the reference patent for patents having different priority dates.[13] The receipt of a statutorily authorized patent term in the earlier-filed, earlier-expiring patent does not raise the risk of gamesmanship, nor is it an unjustified time-wise extension of patent term.[14]
The Board also dismissed a common Examiner rejoinder in these situations, namely that even in the absence of an unjustified time-wise extension of time, the risk of separate ownership necessitates a terminal disclaimer over the later-filed, later-expiring unrelated patent. The Board found that such an argument is “immaterial where the [earlier-filed, earlier-expiring application] is not a proper nonstatutory double patent reference.”[15] This decision should be cited and relied on by patent practitioners when facing this fact pattern.
In summary, the later-filed, later-expiring unrelated patent is not a proper ODP reference patent to an earlier-filed, earlier-expiring patent for at least the following reasons:
- The earlier-filed, earlier-expiring application is “not a second, later expiring patent for the same invention… and as such does not extend a period of exclusivity on the claimed subject matter.” (Baurin, page 10.)
- The USPTO acknowledged the separately patentable nature of the claims in the earlier-filed, earlier-expiring patent by not making an ODP rejection in the later-filed, later-expiring unrelated patent over the earlier-filed, earlier-expiring unrelated patent. (Baurin, page 9.)
- The subject matter of the claims in the later-filed, later-expiring patent could not have been presented in the claims the earlier-filed, earlier-expiring unrelated patent. (Baurin, page 9.)
[1] Ex Parte Baurin, Appeal 2024-002920, Appl. No. 17/135,529 (PTAB Nov. 6, 2024).
[2] Uruguay Round Agreements Act (URAA) modified patent term from 17 to 20 years starting from the earliest non-provisional filing date for applications filed on or after June 8, 1995.
[3] Baurin, page 4, citing Final Office Action, page 6.
[4] In re Cellect, LLC, 81 F.4th 1216 (Fed. Cir. 2023).
[5] Baurin, page 5-6, citing In re Cellect, at 1230.
[6] Gilead Scis., Inc. v. Natco Pharma Ltd., 753 F.3d 1208, 1215 (Fed. Cir. 2014).
[7] Allergan USA, Inc. v. MSN Labs. Private Ltd., 111 F.4th 1358 (Fed. Cir. 2024).
[8] Baurin, page 7, citing Allergan, at 1369.
[9] AbbVie Inc. v. Mathilda & Terence Kennedy Inst. of Rheumatology Trust, 764 F.3d 1366 (Fed. Cir. 2015).
[10] Baurin, page 8.
[11] Baurin, page 9, citing Allergan, at 1371.
[12] Baurin, page 9-10.
[13] Gilead, at 1215-16.
[14] Novartis Pharms. Corp. v. Breckenridge Pharm. Inc., 909 F.3d 1355, 1364 (Fed. Cir. 2018).
[15] Baurin, note 6.

